All About Acrylic Plastic
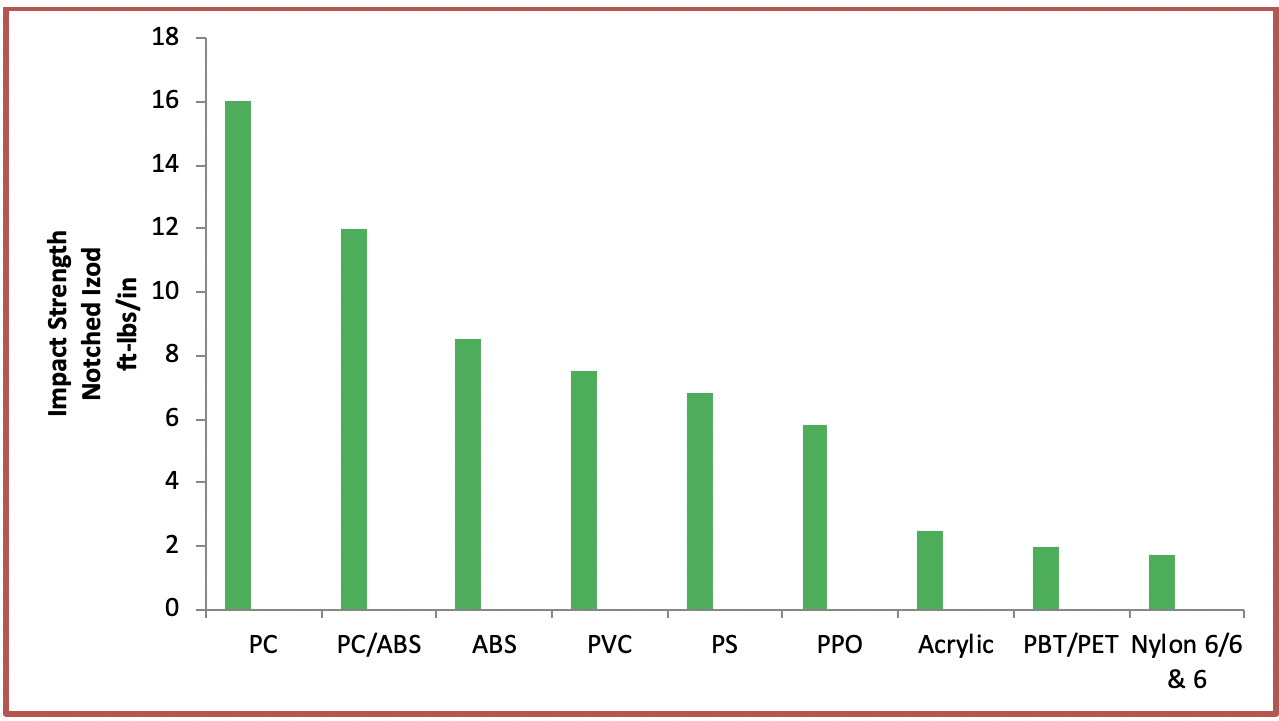
Acrylic is an amazingly useful thermoplastic for a wide range of applications. It has desirable physical properties such as transparency and very good scratch resistance. It has a relatively lower impact strength (as seen in the bar graph below) but a higher impact resistance than glass.
It is often used as a laminated layer over Polycarbonate (PC). Combining it with PC gives a scratch-resistant surface that is very strong and has the high impact resistance of PC. A bullet-proof “glass” is an example of such an application. Acrylic is also lighter than glass, with a density less than half that of glass.
Acrylic is a thermoplastic. Hence, it can be heated and turned into liquid and cooled. It can subsequently be re-heated again and the same process can continue without any significant degradation. Acrylic is inexpensive and readily available.
Acrylic is more economical than Polycarbonate. Another key difference between Acrylic and PC is the fact that Acrylic does not contain bisphenol-A (BPA), which is considered to be a harmful substance. Acrylic is also easy to handle and process. It has a white light transmittance of 92%, which is the highest among any widely used commercial grade material.
History of Acrylic
The earliest record of the discovery of Acrylic can be traced back to the early 1930’s. At that time, a couple of English chemists named Rowland Hill and John Crawford registered, through their company Imperial Chemical Industries, a newly created product under the name of “Perspex”.
Around the same time, a renowned German industrialist and chemist Otto Rohm of the company Rohm and Haas AG also tried making a safety glass by polymerizing methyl methacrylate between two glass sheets. The result of that process was the creation of an acrylic sheet, which ended up separating from the glass. This newly created sheet was given the trade name “Plexiglas”.
With both Perspex and Plexiglas registered under trademarks, commercialization soon followed. Meanwhile, in the United States, DuPont also created its own version of acrylic and registered the product as “Lucite”. World War 2 gave a major boost to the use of acrylic. It was used to make submarine periscopes, fighter airplane windshields, gun turrets, and canopies. Acrylic had health benefits too. Acrylic shards did not damage a pilot’s eyes as much as flying glass shards did when the fighter planes were hit.
Civilian use of acrylic boomed after the war and today, the global demand for acrylic exceeds 6,000 kilo tons per annum.
Manufacturing of Acrylic
Acrylic is formed by a process known as polymerization. In this process, a monomer (often methyl methacrylate) is reacted with a catalyst to create a chain of linked polymers. The catalyst sustains the reaction but does not become a part of the newly formed polymer (acrylic).
There are two types of polymerization processes used to create acrylic: Suspension Polymerization and Bulk Polymerization.
When acrylic is needed in a molding powder form, the process of suspension polymerization is used. In this process, tiny droplets of a monomer are suspended in a solution of catalyst and water. The reaction that subsequently takes place causes grains of acrylic polymer to be formed. This process allows for a tight control of molecular weight. Hence, it is suitable for making a powder form of acrylic.
When acrylic sheets are to be manufactured, then a process known as bulk polymerization is used. In this process, the monomer and catalyst are poured into a mold. The reaction takes place in the mold and the resulting in transparent acrylic sheets.
Within bulk polymerization, there are two sub-types, batch and continuous. Batch bulk polymerization is used for producing acrylic sheets of thicknesses between 0.06 inches to 6 inches. Rods and tubes can be formed using the batch process. The continuous bulk polymerization is less labor intensive and quicker. It is used to make acrylic sheets which are thinner than 0.06 inches.
Further processing of Acrylic
A pure homopolymer form of Acrylic is not usually sold as the end product because its properties are not optimized for commercial use. The pure form needs to be modified by using certain additives and comonomers to customize specific properties as per the ultimate end use. Some examples of this modification are listed below:
- Methacrylic acid is used as a comonomer to increase the transition temperature, making the resulting acrylic suitable for high-temperature environments such as lighting applications.
- Plasticizers are used as additives to improve impact resistance, lower glass transition temperature, or enhance processing properties.
- Butyl Acrylate is also used at times as a comonomer to increase impact strength.
- Dyes are added to provide various colors to acrylic sheets. At times dyes are used to protect the acrylic surface against UV light.
Applications of Acrylic
Medical: Acrylic has a favorable compatibility with the human tissue. Hence, it is used in cosmetic and orthopedic surgeries. It is also used in bioprocess chromatography and more recently in biomedical research to make a microfluidic chip.
Dentistry: Artificial teeth and dentures/dental prosthetics are made using acrylic. This happens to be the case because acrylic teeth will not wear a lot against other teeth and the aesthetics can be controlled to a higher degree as compared to other materials.
Electrical: Plastic optical fiber used for short-range communication is made from PMMA or Acrylic plastic. Certain LCD screens are now being made with acrylic because of its optical properties and its light weight (compared to glass).
Eyewear: Acrylic is used to make contact lenses and eyeglass lenses. Acrylic contact lenses are particularly useful because they help reduce inflammation in cataract surgery patients.
Furniture: Since the 1960s and 1970s, furniture makers have been using acrylic to give furniture a futuristic look. Office chairs and tables are made using translucent or transparent acrylic. Tanning beds also use acrylic.
Fashion accessories: Acrylic has been used to make fashionable bracelets and high heel shoes. Acrylic can be bent, given different colors, and made to give a clean and smooth look. Most recently, nail painting and manicure is done using acrylic.
Sporting venues: Acrylic is used at various stadium and sporting venues for safety and cosmetic value. Ice hockey arenas use acrylic for spectator protection. Stadiums in Europe have used acrylic to build designer canopies.
Police riot gear: Law enforcement agencies use hand-held barriers that are made of acrylic. Riot police vehicles also use acrylic for their windows and windshields. Acrylic is a preferred material due to its transparency and toughness.
Aquariums: Acrylic windows are often seen at residential and commercial aquariums. They are particularly useful in a commercial environment because a thick acrylic window can withstand high water pressure of a tank housing a large fish or a marine animal.
Acrylic Prototypes using CNC and 3D printer machines
CNC machines
Acrylic is available in sheet and round stock forms. It works well with any machining process that involves a mill or lathe. Hence, CNC machines are a good fit with acrylic plastics. Acrylic is transparent and has a reasonable impact resistance, which translates into good machinability.
Post machining, additional processing may be needed. Polishing and sanding may be necessary to remove any cuts marks or scuffs along the edges. Multiple pieces can also be put together using solvents. These solvents melt the acrylic and allow the surfaces to stick together with a barely visible joint, giving a high-quality look and finish.
3D Printing
Acrylic is also available in a filament form which can be used with 3D printers to create prototypes using computer-aided design files. The 3D printer basically heats the acrylic filament and then deposits it according to the design of the prototype. Since acrylic is a thermoplastic material, it can be heated/re-heated and cooled without any major degradation. Various colors of acrylic filaments can be used to 3D print a large variety of models.
Injection molding
Since Acrylic is a thermoplastic, it can be heated, liquefied, and then cooled without any significant degradation. This process can be done over and over. Hence, acrylic can be used in injection molding machines. The raw material feed is normally in the form of granular acrylic. The heated liquid acrylic can then be injected into a mold to take the desired shape.
Disadvantages of Acrylic
- Acrylic has a low impact resistance and strength when compared to Polycarbonate. Hence, it is not the best option for demanding applications.
- Acrylic is not the strongest plastic and is actually quite brittle. Hence, it may develop cracks or break if it is bent even a little bit.
- Acrylic manufacturing and 3D printing using acrylic tends to produce toxic fumes. Any application or process where acrylic is melted tends to produce fumes which should not be inhaled. Proper ventilation and compliance with local laws is needed.
- Acrylic is also not easily recyclable. It belongs to group 7 in the resin identification coding system. This means that it is not bio-degradable and sits in a landfill if it cannot be reused. Only a small proportion of acrylic gets re-used because there should be no cracks or stress on the acrylic surface if it is to be reformed.
Properties and Specs
| Property Type | Detail |
| Scientific Name | Poly(methyl methacrylate) or PMMA |
| Resin Identification Code | Other (7) |
| Chemical Formula | (C5H6 O2)n |
| Tensile Strength | 9400 PSI |
| Glass Transition Temperature (commercial grades) | 85oC – 165oC |
| Specific Gravity | 1.18 |
| Melting Temperature | 150oC |
| Flexural Strength | 90 MPa (13000 PSI) |
| Typical Injection Mold Temperature | 79oC – 107oC |
| Shrink Rate | 0.2 – 1% (0.002 – 0.01 in/in) |
| Notched Izod Impact Strength | 2.5 ft-lbs/in |
| Ignition Temperature | 460oC |
| Maximum Water Absorption | 0.3% to 0.4% |
| Dielectric Constant | 3.06 – 3.73 |
| Heat Deflection Temperature | 95oC at 66 PSI |
| Refractive Index | 1.4905 at 589.3 nm |
| Chemical | Resistance Level |
| Acid (Concentrated) | Poor |
| Acid (Dilute) | Poor |
| Alcohol | Average |
| Alkalis | Good |
| Aromatic Hydrocarbons | Poor |
| Greases and Oils | Good |
| Halogenated Hydrocarbons | Poor |
| Halogens | Poor |
| Ketones | Poor |

